It was Dmitri Mendeleev who first predicted the presence of gallium even before its discovery. Mendeleev named this hypothetical element as eka-aluminium because he had calculated that the element would sit below aluminium in the periodic table.
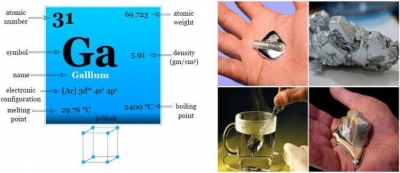
Gallium’s atomic number is 31. French chemist Paul E. Lecoq de Boisbaudran was the person who discovered it through a spectroscope in 1875 in Paris. De Boisbaudran had extracted gallium from a zincblende ore from the Pyrenees. Initially, he had obtained only 0.65 grams from 430 kilograms of ore. But later, he isolated gallium by the electrolysis of gallium hydroxide in potassium hydroxide solution.
Gallium exists as a soft, silvery metal. It is primarily used in electronic circuits, semiconductors, and light-emitting diodes (LEDs). Gallium is also used in high-temperature thermometers, barometers, pharmaceuticals and nuclear medicine tests. This metal is used in electronics in the form of gallium arsenide (GaAs). In fact, nearly 95 per cent of the gallium produced is used to make GaAs, which is a compound used in microwave, semiconductors, and blue and violet LEDs. Gallium is also used in the pharmaceutical industry, particularly radiopharmaceuticals. The radio-active isotope Ga-67 is an isotope of Gallium, which is used as a nuclear medicine test to check for inflammation, or cancer in the body.
Picture Credit : Google




