A small handful of uranium provides as much electrical energy as 70 tons of coal or 390 barrels of oil. A power station big enough to supply a city of a million people consumes just 6.6lb (3kg) of uranium a day, so it is by far the most concentrated source of energy used by man.
Uranium is one of the densest naturally occurring elements and each of its atoms teeters on the edge of instability. The heart of the atom, called the nucleus, needs only a tiny ‘push’ to cause it to divide. And when a nucleus splits it releases huge amounts of energy, in a process called nuclear fission.
The ‘push’ can be provided by neutrons, tiny particles much smaller than atoms, which strike the nucleus and cause it to split. In the process of splitting, at least two extra neutrons are produced, which fly off and cause further fissions – so that once the process has started it can continue almost indefinitely.
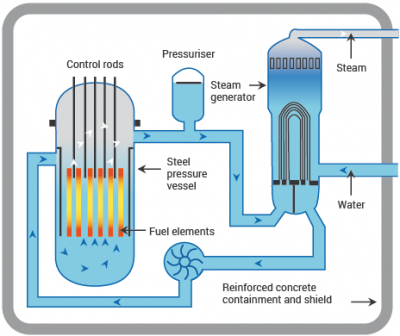
The energy of fission can be released slowly, bit by bit, and used to heat water. The steam from the water is then used to drive a generator, which produces electricity. This is the principle of the nuclear reactor.
Fuel assemblies
Inside most reactors, the fuel assemblies are made from small pellets of uranium dioxide, loaded into thin tubes. The tubes are usually put into vertical bundles with ‘spacers’ to separate them.
Once inside, a fuel assembly may stay there for as long as three years, but even after that length of time, all the uranium has not been consumed. But by-products begin to accumulate; some are gases like krypton, others are solids like caesium, strontium and plutonium. Before these by-products have built up too much, and water corrodes the fuel tubes, the assemblies are removed. To recover the unburned uranium, the spent fuel may be taken to a special plant where it is reprocessed to separate out uranium, plutonium and waste products.
The plutonium is a useful by-product of the nuclear power industry. It can be used as a fuel in power stations, because plutonium, like uranium, has nuclei that can split and release energy.
Uranium occurs in several different forms, identical chemically but with different-sized nuclei in their atoms. Of these different forms, called isotopes, one is uranium-235, which gets its name from the 235 particles making up its nucleus. Only seven atoms out of every 1000 in naturally occurring uranium are U-235. The rest consist almost entirely of uranium-238.
When U-238 is struck by neutrons it does not split as readily as U-235. It may be converted into a completely now element, plutonium-239. So if a reactor is made using natural uranium as fuel, the danger is that too many neutrons will be absorbed by U-238 before they can hit U-235 atoms and cause further fissions. If this happens the reactor will never get going.
There are two ways around this problem. One is to increase the amount of U-235 in the reactor fuel, by a process called enrichment, from seven atoms to between 30 and 40 in every thousand. This is done before the fuel is manufactured, usually in a centrifuge – a machine that whirls round, separating U-235 from U-238 by the outward pushing forces of high-speed rotation. The second way is to make the very best use of the available neutrons inside the reactor by slowing them down, which increases their chances of causing further fissions.
The way to slow them down is to make them ricochet to and fro off light atoms of an element such as hydrogen or carbon, like balls in a pin-ball machine. The light elements act as a ‘moderator’, because their job is to moderate the speed of the neutrons. Most modern reactors use both enriched fuel and moderators. Some are moderated by water (which, of course, contains hydrogen), while others are moderated by carbon in the form of graphite, which is the hard dark material known as the ‘lead’ in an ordinary pencil.
Obviously, a nuclear reactor produces a great amount of heat, and to stop the reactors from overheating, coolants have to be used. Pressurized water reactors use water as a coolant, so these plants need to be built near rivers or oceans. Advanced gas-cooled reactors, first built in Great Britain, are cooled by carbon-dioxide gas. In Canada, heavy water – in which hydrogen atoms are replaced with an isotope of hydrogen called deuterium – cools fast breeder reactors. France has pioneered the use of liquid sodium as a coolant for their fast breeders.
Picture Credit : Google

