How do it rainmakers make it rain?
The Hopi Indians of south west America still trying to make rain by sacrificing Golden Eagles and dancing with live rattlesnakes in their teeth. But while they perform their traditional rain dances to please their gods and get rain for their crops, others have adopted a more scientific approach.
In 1946 Vincent Schaefer and Irving Langmuir started their work at the general electric research laboratories in Schenectady, New York, which proved that rain clouds could be artificially encouraged to produce showers.
Clouds are made up of billions of particles of water too small to fall as rain. Only when the droplets grow to a quarter of a millimetre or more will they fall as a fine drizzle. Smaller droplets evaporate before reaching the ground.
One way the droplets grow is by freezing to form particles of ice. In the cloud containing some ice particles and some water droplets, the ice particles grow rapidly as the droplets evaporate and the valour is transferred to the ice. Since the temperature of clouds is often below freezing it might be expected that the droplets would freeze easily. But the water can be 10 or 20° below freezing (supercooled) without actually freezing.
The reason for this is that the water in clouds is absolutely pure, without any dust or other contaminates which can from the centre of an ice crystal. If tiny particles are provided, the droplets freeze, grow quickly until they are large enough to fall, and then melt as the temperature rises, reaching the ground as rain.
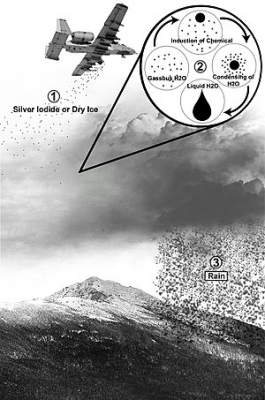
Schaefer and Langmuir proved that small particles, usually of silver iodide, added to supercooled clouds could create rapidly growing ice crystals. These particles have been dropped from aircraft, carried by rockets or even released at ground level for air currents to carry them aloft.
In the Soviet union, 70 mm artillery guns have been used to fire silver iodide particles into clouds, exploding at the right height to disperse the chemical.
As long as the clouds are supercooled the technique may work increasing rainfall by up to a fifth. But since it is impossible to know how much rain could have fallen and anyway there are still question marks over the methods economic effectiveness.
Picture Credit : Google